Although the deadline for compliance with the new sterilisation standards for Australia and New Zealand has been extended, hospitals are still required to have plan in place for achieving compliance by the end of 2021.
How has the timeline has been impacted, what do the changes mean for hospitals; and how can the new requirements most effectively be met? A new standard The new standard, AS/NZS 4187:2014 Reprocessing of reusable medical devices in health service organisations , was released in 2014 and became operational in December 2016. It sets out more stringent requirements for the reprocessing of reusable medical devices in health service organisations, with the aim of making the standards more consistent with European and global standards for sterilisation processes.
The basic requirements for accreditation are that, where reusable equipment, instruments and devices are used, health service organisations should have processes that are consistent with both national and international standards, as well as manufacturer’s guidelines. In addition, a robust traceability process that can identify the patient, procedure, and the reusable equipment, instrument or device that was used for each procedure, must be in place.
Since the Standard was issued, there have been a number of revisions to the related Advisory document, AS18/07 , which sets out the minimum requirements for compliance. The concerns have been mainly around the timeframe, and a revised timeline was released in July 2020. Though there have been discussions about allowing more time, either full compliance or a robust plan and timeframe for reaching compliance will still be required by December 2021. What are the key requirements? As a minimum, there are four key areas within which compliance is essential.
The Advisory states that compliance with the requirements for segregation of clean and dirty activities can be achieved by implementing strategies that ensure sufficient segregation of activities, including unidirectional work and airflows being used to reduce the risk of cross-contamination.
These must be accompanied by a detailed risk analysis and include a process map or flow diagram to indicate how risks of cross contamination are being identified and managed.
All health service organisations are expected to be compliant with requirements to segregate clean and dirty activities by December 2023, through refurbishment and redevelopment of existing sterilising services, endoscopy reprocessing units and satellite services, while all future new builds should be planned to be compliant from the outset.
To meet the AS/NZS 4187:2014 requirements for storage of sterile stock, organisations need to assess the risk of humidity and temperature on stored sterile stock, and ensure risks of contamination are mitigated wherever it is stored. All sterile stock should be stored in compliant shelving, and a risk analysis is required if sterile stock and non-sterile stock are co-located in a storage area.
For organisations that do not currently comply, compliance can be achieved by developing a plan, endorsed by the organisation’s executive, that includes realistic timeframes, costings and options for funding to achieve full compliance by 31 December 2022. In the meantime, a risk assessment, including mitigation strategies, and evidence of a regular review process will need to be prepared.
Whenever an organisation replaces cleaning, disinfecting and/or sterilising equipment, it must install equipment that is compliant with AS/NZS 4187:2014 and the relevant applicable ISO standard. The equipment should also be operated and maintained in accordance with the standard and the manufacturer’s requirements, including for water quality and water monitoring.
Equipment and design of all new builds, refurbishments and redevelopments of sterilising services units are required to comply with the Standard by 31 December 2022. But compliance can also be achieved by developing a detailed plan that includes realistic timeframes, costings and options for funding to move to full compliance. In this case, a risk assessment, and evidence of regular review and reports to the executive on progress, must be in place by 31 December 2021.
Health service organisations are required to comply with water monitoring requirements for all reprocessing equipment as well as the applicable ISO standards. Compliance is required by 31 December 2022 but can also be achieved by submitting a detailed plan, by 31 December 2021.
Whenever an organisation replaces equipment that is used in the cleaning, disinfecting or sterilisation process, water quality requirements should also be included in the planning and risk assessment.
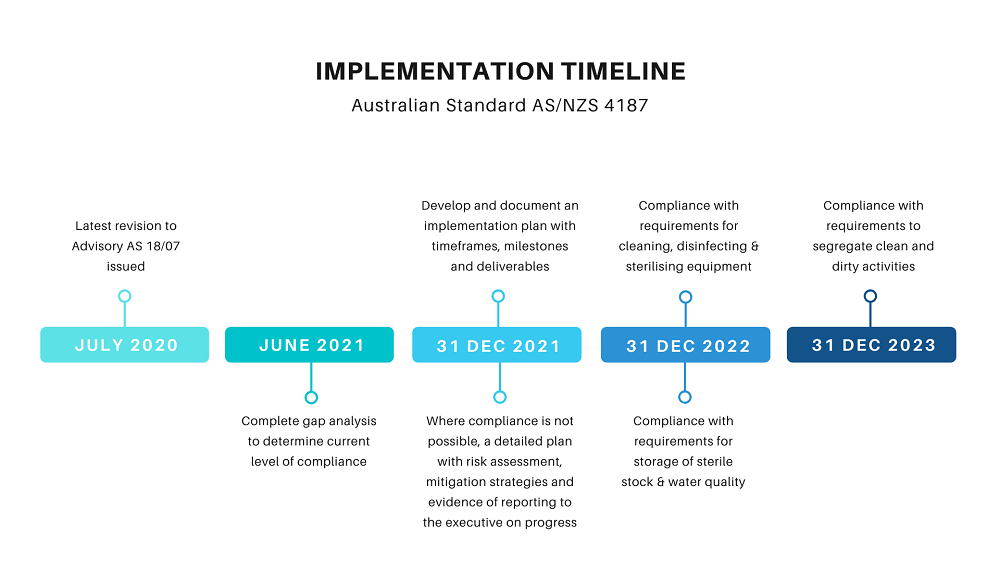 Source: Advisory AS18/07 – July 2020 update
What it means for hospitals
It is mandatory for approved accrediting agencies and health service organisations to implement the new standard. Accreditation is awarded on a three or four-year cycle, depending on the accrediting agency.
Source: Advisory AS18/07 – July 2020 update
What it means for hospitals
It is mandatory for approved accrediting agencies and health service organisations to implement the new standard. Accreditation is awarded on a three or four-year cycle, depending on the accrediting agency.
While individual health departments regulate which services must undertake accreditation, all Australian States and Territories have agreed that hospitals and day procedure services should be accredited to the NSQHS Standards from January 2013 onwards. This is also a requirement for private health insurance.
In practice, the need for segregating clean and dirty activities may mean redesigning floor space and physically segregating the reprocessing environment, which in turn could mean more space is needed. Hospitals will also need to review conditions in existing storage facilities, such as temperature, humidity and contamination risk, and if facilities are found to not be compliant, this could mean re-design of the facility or investment in upgrading air conditioning systems is required.
In terms of equipment needed, it is expected that many hospitals will need to replace or invest in new equipment for their central sterile services departments, including washer disinfectors (compliant with ISO 15883) and sterilisers (compliant with EN 285 or EN 13060).
However, simply replacing the equipment is not enough; wider changes to processes are likely to be needed as operational complexity increases. In practice, health service organisations will need to develop procedures for operational use of the equipment that are compliant with the standard, and training may also be required. This is likely to mean a higher level of expertise will be required, costing time, money and resources.
The new standard also requires that instruments are traceable to the patient. A tracing system, ideally an electronic system, is required that can identify the cleaning process applied to each reusable medical device, including an ability to track a device to a patient to allow for a recall if required. What are the key challenges? When the new standard was first introduced, reports in the media suggested that Australian hospitals may face an estimated $1 billion cost to overhaul central sterilisation departments to meet the new requirements.
The trigger for the most recent set of revisions was a workshop held by the Australian Commission on Safety and Quality in Health Care in September 2019 with participants from both the public and private sectors, specifically to discuss the issues health service organisation had identified with the implementation of the new standard.
A survey by the Commission had found that, while over 90% of respondents had completed a gap analysis as required, only 30% of organisations reported compliance with the standard, and 50% expected to be compliant by 2021. As many as 90% of all organisations also identified implementation issues.
Workshop participants recognised the need for updating the standards and that current sterilisation practices do not meet best practice, but had identified a need for simplified guidance and clearer documentation to assist in accurately interpreting and applying the standards, as well as improved access to training. It was also recommended that a risk-management approach to implementation be adopted to enable prioritisation of high-risk areas, and this has influenced the more lenient wording of the latest revision to the Advisory.
From a management perspective, the largest challenges for health service organisations in meeting the AS/NZS 4187:2014 standard is likely to be; limitations to capital and ability to fund major refurbishments; and physical capacity, or space available. The cost of new equipment or infrastructure upgrades to improve air and water quality, or implement an electronic tracing system, can be substantial.
For some hospitals, the requirement for separating all dirty and clean activities is likely to mean the sterile services department may need to be expanded and, in some cases, will require more space than is currently available. Additional space may also be needed for new equipment or to meet the new demands for the correct storage of sterile devices.
Since it is critical that the hospital has access to sterilised equipment at all times, and that sterilisation and reprocessing services are not disrupted, undertaking refurbishment works also presents an operational challenge in the short to medium term.
The revised timescale that was recently announced will be welcomed. It allows for more time to implement robust, strategic plans for major refurbishment work, rather than a need to opt for an interim solution in order to achieve compliance. However, implementing the changes will provide a challenge and appropriate compliant facilities, such as Q-bital Healthcare Solutions’ mobile CSSD units, where activities can continue without disruption, are essential while these changes take place.


Q-bital Healthcare Solutions
Unit 1144 Regent Court, The Square, Gloucester Business Park, Gloucester, GL3 4AD
