Refurbishing the sterilisation or decontamination departments of a hospital - whether it is planned or the result of an emergency - can be a challenging project. Additional space will probably be needed to set up a temporary facility, and the entire flow of instruments and endoscopes within the hospital risk being disrupted.
Sterilisation and decontamination departments are often in use seven days a week, and are critical for the treatment of patients and for procedures to carry on uninterrupted. It is also essential that decontamination of any instrument used in a hospital setting meets the latest standards, such as AS/NZS 4187:2014, and this can be difficult to achieve with a temporary setup.
Increasing demand on healthcare services has led to a growing number of instruments making their way through sterilisation and decontamination departments. This, in turn, is leading to increased ‘wear and tear’ on the specialist - and expensive - equipment needed to carry out the tasks, and sterilisation equipment having a shorter lifespan, meaning it needs to be replaced more often.
Ensuring continuity and compliance
Hospitals without the space or the budget to reconstruct a new or temporary internal sterilisation or decontamination department have limited options. Using a neighbouring hospital’s sterilisation facilities temporarily, or outsourcing the whole process, means sending instruments to an off-site location.
This means the hospital could be faced with delays in getting vital instruments back to the department, an increased risk of contamination and the hospital may also need to buy more instruments to cover the increased ‘downtime’. In the case of endoscopes this could prove a very expensive option.
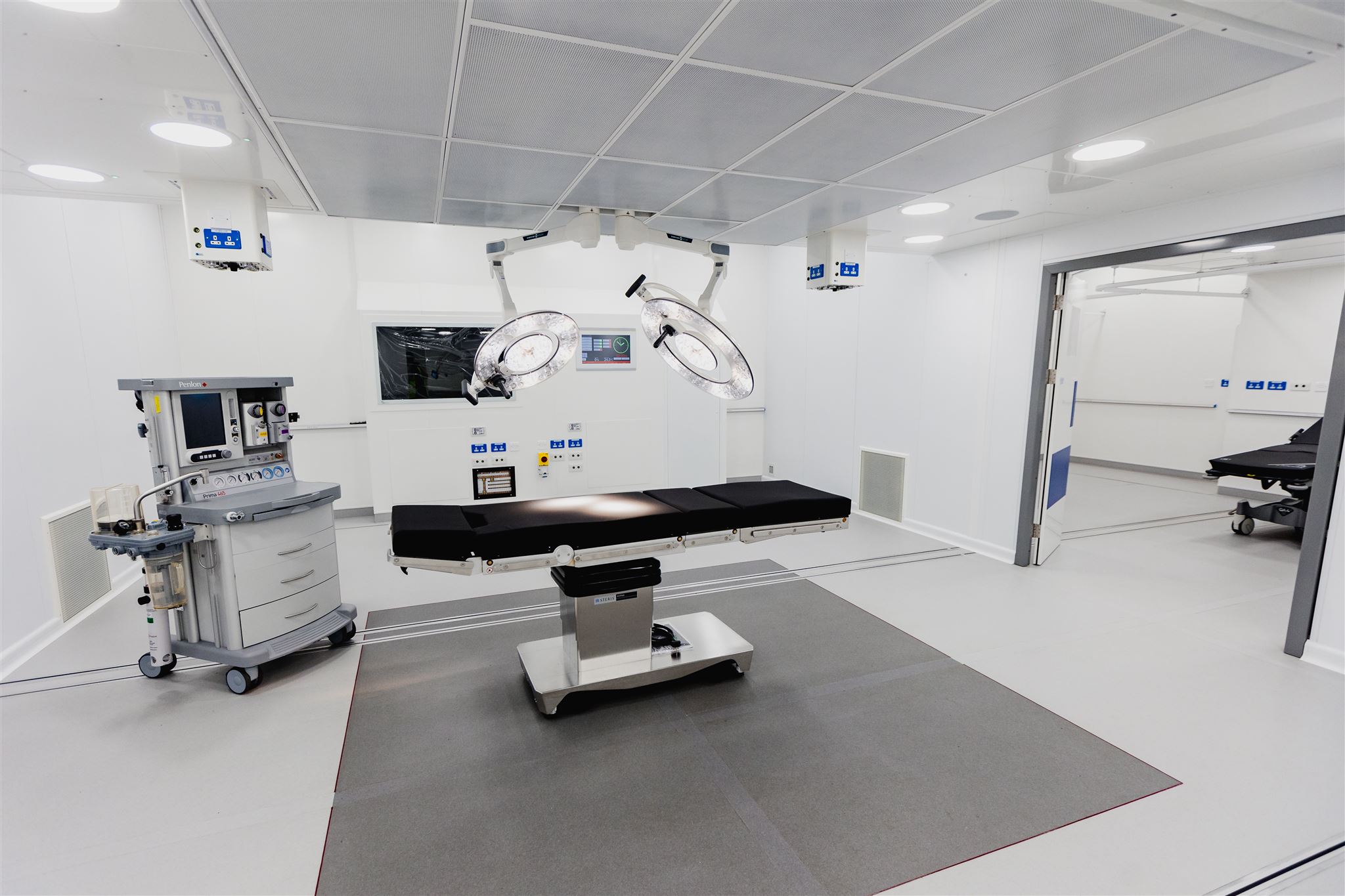
Another other option is to deploy a flexible, mobile or modular solution which allows cleaning and sterilising to continue take place on-site. One example is Q-bital’s mobile Central Sterile Services Department (CSSD) unit, which arrived in Australia last month.
Fully integrated and designed to provide replacement capacity, it can help hospitals to continue delivering the vital service of cleaning, sterilising and repackaging of surgical instruments during a temporary disruption or exceptional demand.
Keeping sterilisation on site
The mobile CSSD units offered by Q-bital are compliant with the latest Australian standards, and can be brought to any hospital site, allowing all processes to continue uninterrupted; sterilisation activity to be kept on site; and the hospital’s existing staff to remain in control of the process.
The sterilisation process is strict for surgical instruments. First, a manual clean is required, followed by a stint in the washer disinfector. Then the instruments are packaged and wrapped, steam sterilised and placed on a cooling rack. The water used in the sterilisation process needs to be purified and disinfected, and the air in the department needs to be filtered to ensure optimal cleanliness.
Q-bital’s CSSD unit, which is completely stand-alone, takes care of all this and contains all facilities and equipment needed for the process. Units contain an integrated RO system with water softener and brine tank to ensure water quality meets all requirements, and provides HEPA-filtered environmental air. Integration with the hospital’s own track and trace system is also provided, allowing the hospital to retain control over the flow of instruments.
Mobile sterilisation departments have one-way flow and incorporate a pre-cleaning area with a manual clean workbench, washer disinfectors and steam sterilisers, a clean packing room and a post sterilisation processing room, as well as dirty and clean utility areas. A staff changing area, including WC and hand washing facilities, is also provided for the comfort of staff working in the unit.
Endoscope decontamination
Endoscopes need even more specialist cleaning in dedicated endoscope decontamination units which are specially equipped and use a high disinfectant washing process and purified water. Mobile or modular endoscope decontamination units are also available to support hospitals that want to keep this activity on site at all times.
Q-bital’s flexible endoscope decontamination solution provides separate clean and dirty staff and endoscope flows. It includes inbuilt IP65-rated equipment for the hospital’s own track and trace system, Duplex Reverse Osmosis water system, cabinets and sinks, as well as a staff welfare area with WC and a technical room.
The facility is compliant with the latest standards, including for fire safety, and can process up to 120 scopes per day. It provides a spacious, climate-controlled work environment with natural light, and has been designed and equipped in consultation with frontline staff.
What preparations are needed?
A flexible mobile CSSD or endoscope decontamination facility can be installed very quickly with a minimum of preparation, depending on the characteristics of the site. All that is needed is a relatively flat hard standing area, such as a car park or concrete pad, along with connections to utilities, which need to be within a reasonable distance from where the unit will be placed.
The process for providing a solution starts with a free, no-obligation site survey. We will assess space, access, clinical adjacency, services, risk, and other issues critical to providing hospitals with an analysis of possible solutions.
Water validation, electrical testing and validation of washers and sterilisers form an essential part of the commissioning and validation process that takes place once the unit has arrived on site. Q-bital works closely with the hospital throughout the commissioning phase and provides staff training and an induction on the unit, which includes ‘sign-off’ of hospital staff.
For many hospitals, achieving compliance with the new AS/NZS 4187:2014 sterilisation standards will mean remodelling or upgrading the central sterilisation department, and bringing a fully compliant mobile CSSD unit on-site will enable this to be achieved with a minimum of disruption.
Get in touch on info@q-bital.com to find out more about our mobile CSSD and endoscopy decontamination unit offers and current availability in Australia.


Q-bital Healthcare Solutions
Unit 1144 Regent Court, The Square, Gloucester Business Park, Gloucester, GL3 4AD
